i3- structure hybridization|Iba pa : Pilipinas I3- molecular geometry is linear. While there are three Iodine atoms, one of the atoms has a negative charge which further gives 3 lone pairs of electrons and 2 bond pairs. Its steric number will be 5. The three lone pairs will repel each other and take up . Tingnan ang higit pa TRB: Dennis Rodman (247) AST: John Stockton (195) WS: Michael Jordan (4.7) More playoffs info. Become a Stathead & surf this site ad-free. 1996 NBA Playoffs; Standings; . D. Rodman-CHI: 19: Playoff Series. Four Factors. Chicago Bulls (4-2) Chicago Bulls Advanced Stats. Seattle SuperSonics (2-4) Seattle SuperSonics Advanced Stats.
PH0 · lone pairs in i3
PH1 · i3 molecular geometry
PH2 · i3 minus structure
PH3 · i3 lewis structure
PH4 · i3 hybridization of central atom
PH5 · Iba pa
Minato Namikaze, also known as the Fourth Hokage, is a legendary Shinobi from the Hidden Leaf Village in the Naruto universe. He is the father of Naruto Uzumaki and the husband of Kushina Uzumaki. Minato's life is intertwined with the powerful Nine-Tailed Fox, Kurama, which plays a significant role in his story and his son's life.
i3- structure hybridization*******Learn how to calculate the hybridization of I3- ion, a linear anion formed by the bonding of I2 with I- ion. Find out the number of valence electrons, lone pairs, bond pairs, and steric number of I3- and its molecular geometry and bond angles. Tingnan ang higit paTo know the hybridizationof Triiodide ion, we can use simple hybridization formula which is given as; If we look at the iodine atoms there are seven valence electrons in its outer shell and two monovalent atoms are also present. Further, during the combination . Tingnan ang higit paI3- molecular geometry is linear. While there are three Iodine atoms, one of the atoms has a negative charge which further gives 3 lone pairs of electrons and 2 bond pairs. Its steric number will be 5. The three lone pairs will repel each other and take up . Tingnan ang higit pa Learn how to draw the Lewis structure of I3-, a triiodide ion with a negative charge, and how to calculate its hybridization, polarity, and molecular geometry. Find out the steps, formulas, and examples for .
It is important to know the Lewis structure of a molecule to understand its physical properties, hybridization, and shape of the .i3- structure hybridization I3⁻ exhibits a linear geometry with bond angles of 180°, consistent with sp³d hybridization. The presence of the extra electron on the central iodine contributes to the .
This chemistry video tutorial explains how to draw the lewis structure of I3-. It also discusses the molecular geometry, bond angle, hybridization, and formal charges . I3 (-) Hybridization is a term used to describe the process of combining two or more. In this section, we'll learn how to determine the Hybridization of I3-. Learn how to calculate the hybridization of I3-, a linear anion formed by the bonding of I2 and an I− ion. Find out the molecular geometry, bond angle and lone pairs .
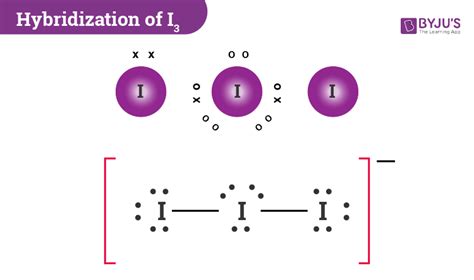
I quickly take you through how to draw the Lewis Structure of I3- (TriIodide Ion). I also go over hybridization, shape and bond angle.I 3- is dsp 3 hybridized and contains 3 lone pairs and 2 bonding pairs of valence electrons around the Iodine. The VSEPR predicts the linear shape. Elements in the first 2 periods . The simplest answer is distribution of charge. Rather than have a single negative charge on one atom you end up with a structure that has approximately half a negative charge on two . This indicates that the hybridization of I3- is sp 3 d. Another way to determine the hybridization of I3- is by counting the number of valence electrons and lone pairs and adding them together. In this case, we have 3 lone pairs and 2 atoms donating valence electrons, giving us a total of 5, which also indicates sp 3 d hybridization.i3- structure hybridization Iba pa This chemistry video tutorial explains how to draw the lewis structure of I3-. It also discusses the molecular geometry, bond angle, hybridization, and form.

Geometrical isomers. For some molecules in the Table, we note that there is more than one possible shape that would satisfy the VSEPR rules. For example, the XeF 2 molecule has a steric number of five and a trigonal bipyramidal geometry. There are three possible stereoisomers: one in which the F atoms occupy axial sites, resulting in linear . About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright . I3- ion has sp3d hybridization and linear shape containing two bond pair and three lone pair while I3+ ion has sp3 hybridization and bent shape containing two bond pair and two lone pair. Related Questions: . The structure of crystalline solids is determined by packing of their constituents .In order to understand the packing of the .Iba pa5.3: Hybridization of Atomic Orbitals. Page ID. Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. It is experimentally observed that bond angles in organic compounds are close to 109°, 120°, or 180°. According to Valence Shell Electron Pair Repulsion ( VSEPR) theory .
Steps. Use these steps to correctly draw the I 3– Lewis structure: #1 First draw a rough sketch. #2 Mark lone pairs on the atoms. #3 Calculate and mark formal charges on the atoms, if required. Let’s discuss each step in more detail.In chemistry, triiodide usually refers to the triiodide ion, I − 3.This anion, one of the polyhalogen ions, is composed of three iodine atoms. It is formed by combining aqueous solutions of iodide salts and iodine.Some salts of the anion have been isolated, including thallium(I) triiodide (Tl + [I 3] −) and ammonium triiodide ([NH 4] + [I 3] −).Triiodide is . The central atom undergoes sp 2 hybridization and the only covalent molecule can undergo hybridization. The ionic molecule is made by lattice crystal structure which is also shown in the AlI 3, so it can be thought of as ionic. The cation Al 3+ has a higher ionic potential (charge density high) so it can be polarized by many anions. .
Q. According to the VSEPR theory, the geometry and shape of the molecule depends upon: Q. Using vsepr theory, predict the geometry of XeF^+5 ion. Q. The geometry of ClO− 3 ion according to Valence Shell Electron Pair Repulsion (VSEPR) theory will be: Q. Based on VSEPR theory predict the geometry and shape of : a) ClF 3. An explanation of the molecular geometry for the I3 - ion (Triiodide Ion) including a description of the I3 - bond angles. The electron geometry for the Trii. The Lewis structure of XeO3 helps us visualize the arrangement of atoms and electrons in the molecule. It is a diagram that shows the bond ing and nonbonding electrons around the central atom, xenon. To determine the Lewis structure of XeO3, we first need to know the total number of valence electrons in the molecule. We’ll talk about NF3 lewis structure, formal charges, geometry, hybridization and uses of NF 3 in this article.. Nitrogen trifluoride (NF 3) is an inorganic, colourless, non-flammable, toxic gas with a slightly musty odour.In the NF 3 molecule, nitrogen is attached to three fluorine atoms via a single bond and has a molecular weight .The observed structure of the borane molecule, BH 3, suggests sp 2 hybridization for boron in this compound. The molecule is trigonal planar, and the boron atom is involved in three bonds to hydrogen atoms ( Figure \(\PageIndex{7}\)). Figure \(\PageIndex{7}\): BH 3 is an electron-deficient molecule with a trigonal planar structure.
I quickly take you through how to draw the Lewis Structure of I3- (TriIodide Ion). I also go over hybridization, shape and bond angle.
CH3CN Polarity. Polarity is the existence of two opposite poles in a molecule i.e. a positive and a negative. These poles develop due to the difference in electronegativity of the bonding atoms amongst which the more electronegative atom attracts the electron more towards itself, thus developing a slight negative charge, while the other atom . IO3- Lewis Structure Hybridization. Hybridization is the process in which hybrid orbitals are formed by mixing of same energy atomic orbitals. The ground state outermost shell electronic configuration of I is 5s 2 5p 5. As we see from electronic configuration of I atom that there is only 1 unpaired electron and to form io3- ion 3 . 1.6.2 Hybridization and the Structure of CH 4. Simply speaking, hybridization means the mathematical combination of several orbitals to generate a set of new hybrid orbitals. In the hybridization for CH 4, the 2s and three 2p orbitals are combined to give a new set of four identical orbitals, that are called sp 3 hybrid orbitals.
The indoor parking facilities are covered and provided with professional security and electronic surveillance. The company is open 24 hours a day, 365 days a year, Sundays and holidays included. The company has a comfortable lounge where customers are able to wait for their flight or while their cars undergo repairs and/or cleaning.
i3- structure hybridization|Iba pa